Spatiotemporal Decoupling of Water Electrolysis for Dual-Use Grid Energy Storage and Hydrogen Generation
Electrolysis system that could reduce the costs of hydrogen produced by electrolysis by using a cerium redox mediator to decouple water splitting, resulting in a system that can arbitrage fluctuating renewable electricity prices.
This research is led by Miguel Modestino, assistant professor of chemical and biomolecular engineering and Yury Dvorkin, assistant professor of electrical and computer engineering.
Increased efforts to curb global warming have led to a drastic surge in the deployment of renewable electricity sources, such as wind and solar power. However, as these sources form a larger fraction of the energy in the grid, their intermittency has started to cause supply instability and large fluctuations in energy prices.
To mediate this challenge, electrochemical energy storage devices such as lithium ion batteries have started to enter the utility scale market. Also gaining traction are redox flow batteries (RFB) because of their long cycle life and power and energy capacity. Despite their promising performance RFB costs remain high due to low energy density of redox energy carriers, which results in large operational costs.
An alternative energy storage method is the production of energy-dense electrofuels, such as hydrogen (H2). H2 has gained significant attention as a promising energy vector for a renewable-rich energy future, given its high gravimetric energy density that makes it desirable for both stationary and mobile applications.
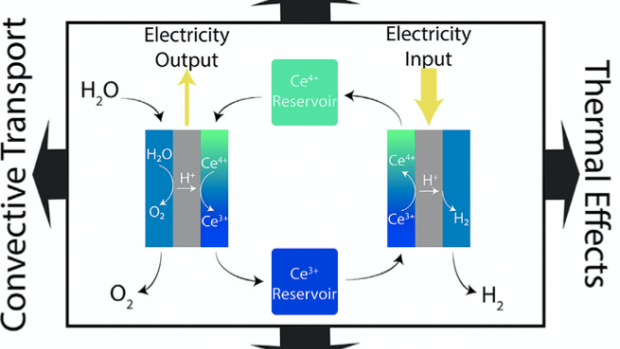
To make electrochemical hydrogen production more competitive, renewable energy sources need to be used with new strategies for electrochemical hydrogen production. The researchers, including Ph.D. students Daniel Frey (lead author) and Jip Kim, show a cerium-mediated decoupled electrolysis system that produces hydrogen and stores energy in the redox couples. They present electrochemical studies to observe the effects of diffusive transport, convective transport, and thermal effects. Following this, the team performed a techno-economic analysis, focusing on the optimization of the system operation and the identification of target operation parameters to achieve hydrogen production at a competitive price.