Brain-Muscle Connectivity Network for Assessing Stroke NeuroRehabilitation
S. Farokh Atashzar of NYU Tandon, and John-Ross Rizzo of NYU Langone Health and NYU Tandon are pioneering a system that tracks and computationally models CorticoMuscular function and connectivity, and builds the basis for the first medical device development tool for systematic, objective and transparent evaluation of pre-market rehabilitation devices for stroke.
This project is led by S. Farokh Atashzar, assistant professor of electrical and computer engineering at NYU Tandon; and John-Ross Rizzo, assistant professor in the Departments of Rehabilitation Medicine and Neurology at NYU Langone Health, and of mechanical and aerospace and biomedical engineering at NYU Tandon.
Stroke, the leading cause of motor disabilities, is putting tremendous pressure on healthcare infrastructures because of an imbalance between an aging society and available neurorehabilitation resources. Thus, there has been a surge in the production of novel rehabilitative technologies for accelerating recovery. Despite the successful development of such devices, lack of objective standards besides clinical investigations using subjective measures have led to controversial recommendations regarding several devices, including robots.
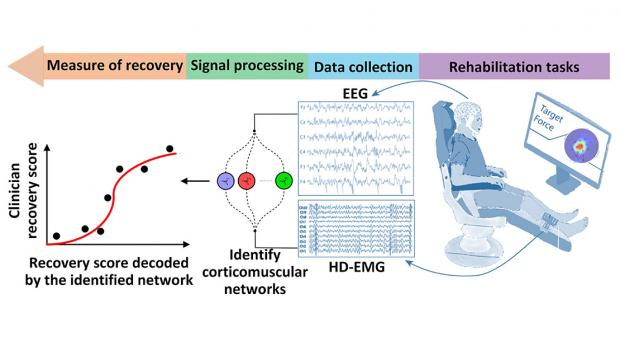
This NSF/FDA Scholar-in-Residence project, designed to address the need for effective rehabilitative technologies, is focused on the design, implementation, and evaluation of a novel, objective, and robust algorithmic biomarker of recovery. Called Delta CorticoMuscular Information-based Connectivity (D-CMiC), the proposed algorithm-based protocol quantifies the connectivity between the central nervous system (CNS) and the peripheral nervous system (PNS) by simultaneously measuring electrical activity from the brain and an ankle muscle on the affected side of recovering post-stroke patients. The system will quantify both spectrotemporal neurophysiological connectivity between the CNS (using electroencephalography (EEG) and PNS (using high-density surface electromyography (HD-sEMG).
The goal of the collaborative project, beyond clarifying the neurophysiology of recovery, is to expedite availability of more effective rehabilitation devices to patients for a range of neurological disorders beyond stroke (such as Parkinson's disease, Essential Tremor and Ataxia). For educational impact, the project will generate a unique transdisciplinary educational environment by conducting workshops about emerging Brain-Computer Interface (BCI) technologies in medicine, and undergraduate team projects for human-machine interfacing, with a focus on promoting STEM activities within underrepresented groups.
The predictive capability, precision, and efficiency of the developed D-CMiC metric will be analyzed by collecting data from recovering stroke patients and healthy subjects alike. Unique D-CMiC features include: (1) accurately and objectively tracking corticomuscular functional connectivity in the Delta/low frequency band; (2) computationally modeling of corticomuscular connectivity; (3) building the basis for the first medical device development tool for the systematic, objective, and transparent evaluation of pre-market rehabilitation devices, aligned with the FDA's mission.